- +86-0533-2185556
- +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >112887-68-0

pd_meltingpoint:176-180 °C
Appearance:yellow crystalline powder
Purity:99%
|
Biochem/physiol Actions |
Raltitrexed is a folate-based inhibitor of thymidylate synthase (TS) that is rapidly and extensively metabolized to its more potent polyglutamate derivatives. By inhibiting the formation of precursor pyrimidine nucleotides, raltitrexed prevents the formation of DNA and RNA, which are required for the growth and survival of both normal cells and cancer cells. |
|
Drug interactions |
Potentially hazardous interactions with other drugs Antipsychotics: avoid with clozapine, increased risk of agranulocytosis. Folic and folinic acid: impairs cytotoxic action - avoid. |
|
Metabolism |
Raltitrexed is actively transported into cells and metabolised to active polyglutamate forms. The remainder of a dose is not metabolised and is excreted unchanged, about 50% of a dose appearing in the urine, and about 15% in the faeces. |
|
Brand name |
Tomudex (Zeneca). |
InChI:InChI=1/C20H24N4O2S/c1-12-21-15-7-6-13(10-14(15)18(25)22-12)11-24(5)17-9-8-16(27-17)19(26)23-20(2,3)4/h6-10H,11H2,1-5H3,(H,23,26)(H,21,22,25)
The quinazoline-based inhibitor of thymi...
The invention belongs to the field of dr...
The invention relates to a raltitrexed p...
The invention belongs to the field of me...
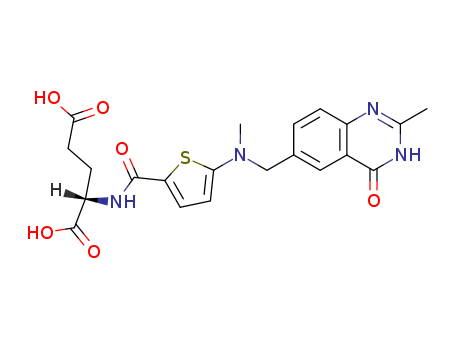
![N-[[5-[N-(3,4-dihydro-2-methyl-4-oxo-6-quinazolinyl)methyl]-N-methyl]amino-2-thiophenecarbonyl]-L-glutamic acid diethyl ester](/upload/2025/4/0c727520-3e92-447f-921a-143f90c9c4d1.png)
N-[[5-[N-(3,4-dihydro-2-methyl-4-oxo-6-quinazolinyl)methyl]-N-methyl]amino-2-thiophenecarbonyl]-L-glutamic acid diethyl ester

![N-[5-[N-[(3,4-dihydro-2-methyl-4-oxy-6-quinazolinyl)-methyl]-N-methylamino]-2-thenoyl]-L-glutamic acid](/upload/2025/4/ac6fd1b3-ffe6-4b0a-a49e-e6465ea766d6.png)
N-[5-[N-[(3,4-dihydro-2-methyl-4-oxy-6-quinazolinyl)-methyl]-N-methylamino]-2-thenoyl]-L-glutamic acid
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In ethanol; at 20 ℃; for 2h;
|
88.6% |
|
With sodium hydroxide; In water; at 25 ℃;
|
80% |
|
With sodium hydroxide; at 20 ℃; for 2h;
|
80.6% |
|
With water; sodium hydroxide; at 0 - 15 ℃; for 2h;
|
77.6% |
|
With sodium hydroxide; for 2h;
|
41% |
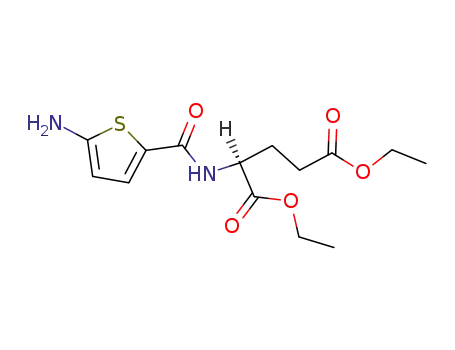
N-(5-aminothiophene-2-carbonyl)-L-glutamic acid diethyl ester

![N-[5-[N-[(3,4-dihydro-2-methyl-4-oxy-6-quinazolinyl)-methyl]-N-methylamino]-2-thenoyl]-L-glutamic acid](/upload/2025/4/ac6fd1b3-ffe6-4b0a-a49e-e6465ea766d6.png)
N-[5-[N-[(3,4-dihydro-2-methyl-4-oxy-6-quinazolinyl)-methyl]-N-methylamino]-2-thenoyl]-L-glutamic acid
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: 48 percent / 2,6-lutidine / dimethylformamide; toluene / 24 h / 70 °C
2: 22 percent / 2,6-lutidine / various solvent(s) / 18 h / 80 °C
3: 41 percent / 1 N aq. NaOH / 2 h
With 2,6-dimethylpyridine; sodium hydroxide; In N,N-dimethyl-formamide; toluene;
|
|
|
Multi-step reaction with 3 steps
1.1: ethyl acetate / 24 h / 50 °C
1.2: 5 h / 0 - 20 °C
2.1: sodium hydrogencarbonate / N,N-dimethyl-formamide; chloroform / 12 h / Reflux
3.1: sodium hydroxide / water / 25 °C
With sodium hydrogencarbonate; sodium hydroxide; In chloroform; water; ethyl acetate; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 4 steps
1: 2 h / 80 °C
2: sodium tetrahydroborate; methanol / 2 h / 0 - 20 °C
3: sodium hydrogencarbonate / N,N-dimethyl-formamide; chloroform / 12 h / Reflux
4: sodium hydroxide / water / 25 °C
With methanol; sodium tetrahydroborate; sodium hydrogencarbonate; sodium hydroxide; In chloroform; water; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 4 steps
1: 2 h / 80 °C
2: sodium tetrahydroborate; ethanol / 2 h / 0 - 20 °C
3: sodium hydrogencarbonate / N,N-dimethyl-formamide; chloroform / 12 h / Reflux
4: sodium hydroxide / water / 25 °C
With sodium tetrahydroborate; ethanol; sodium hydrogencarbonate; sodium hydroxide; In chloroform; water; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 4 steps
1: 2 h / 90 °C
2: sodium tetrahydroborate / tetrahydrofuran / 2 h / 0 - 20 °C
3: sodium hydrogencarbonate / N,N-dimethyl-formamide; chloroform / 12 h / Reflux
4: sodium hydroxide / water / 25 °C
With sodium tetrahydroborate; sodium hydrogencarbonate; sodium hydroxide; In tetrahydrofuran; chloroform; water; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1: pyridine; 2,6-dimethylpyridine / N,N-dimethyl-formamide / 12 h / 45 - 52 °C
2: 2,6-dimethylpyridine / N,N-dimethyl-formamide / 12 h / 50 - 55 °C
3: sodium hydroxide / 2 h / 20 °C
With pyridine; 2,6-dimethylpyridine; sodium hydroxide; In N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 5 steps
1: 24 h / 10 - 25 °C
2: sodium carbonate / N,N-dimethyl-formamide / 2 h / 80 °C
3: sodium methylate; methanol / 1 h / 10 - 25 °C
4: sodium hydrogencarbonate / N,N-dimethyl-formamide / 3 h / 70 °C
5: sodium hydroxide; water / 2 h / 0 - 15 °C
With methanol; water; sodium methylate; sodium hydrogencarbonate; sodium carbonate; sodium hydroxide; In N,N-dimethyl-formamide;
|
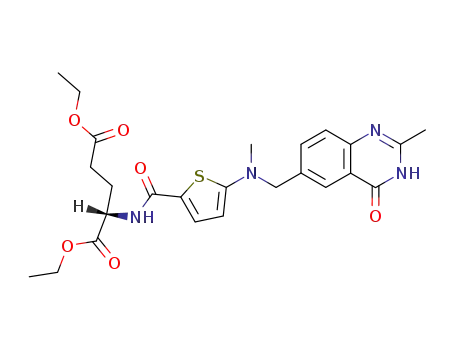
N-[[5-[N-(3,4-dihydro-2-methyl-4-oxo-6-quinazolinyl)methyl]-N-methyl]amino-2-thiophenecarbonyl]-L-glutamic acid diethyl ester
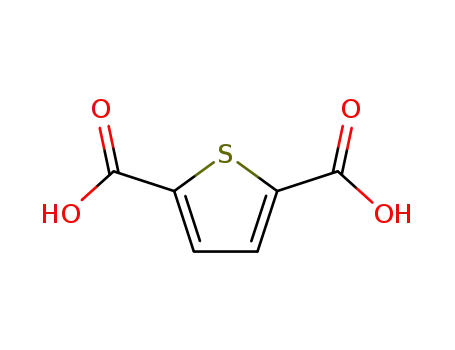
Thiophene-2,5-dicarboxylic acid
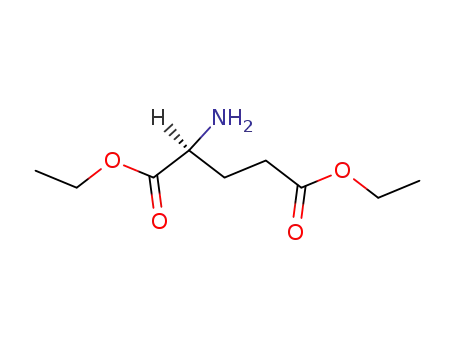
L-glutamic acid diethyl ester
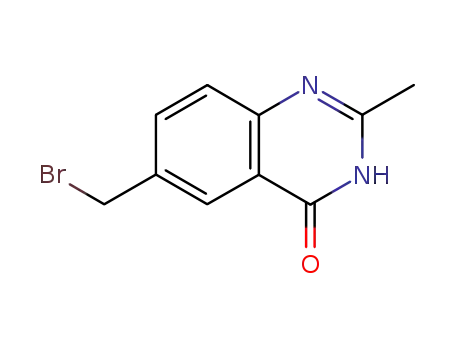
4(3H)-oxo-6-(bromomethyl)-2-methyl quinazoline
CAS:115473-15-9
CAS:1173-88-2
CAS:6422-84-0
CAS:1097796-78-5