- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >2306-27-6
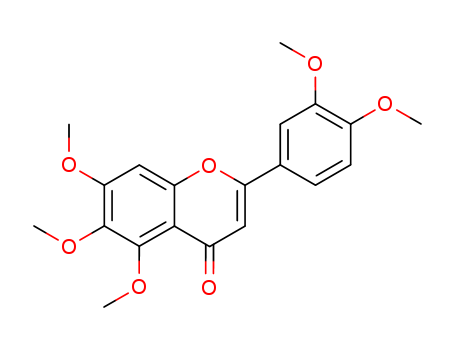

pd_meltingpoint:174-176°C
Appearance:pale creamy yellow powder
Purity:99%
|
Biochem/physiol Actions |
Sinensetin is a citris flavonoid with anti-inflammatory and anti-proliferative activity. It has also been shown to enhance adipogenesis and lipolysis. |
|
Definition |
ChEBI: A pentamethoxyflavone that is flavone substituted by methoxy groups at positions 5, 6, 7, 3' and 4' respectively. |
|
General Description |
This substance is a primary reference substance with assigned absolute purity (considering chromatographic purity, water, residual solvents, inorganic impurities). The exact value can be found on the certificate. Produced by PhytoLab GmbH & Co. KG |
InChI:InChI=1/C20H20O7/c1-22-13-7-6-11(8-15(13)23-2)14-9-12(21)18-16(27-14)10-17(24-3)19(25-4)20(18)26-5/h6-10H,1-5H3
5,6-Dimethoxy-7,3',4'-trihydroxyflavone ...
-
A series of 5,6,7-trimethoxyflavones 1a-...
The invention discloses a nobiletin deri...
Disclosed is a use of flavones derivativ...
Previous investigations of the aerial pa...
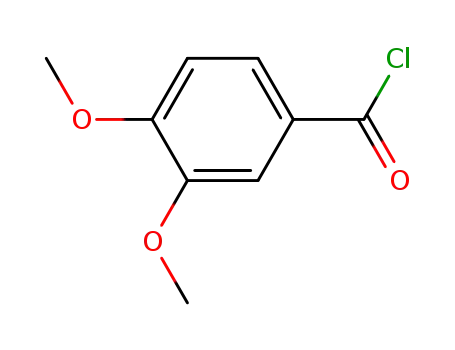
3,4-dimethoxybenzoic acid chloride

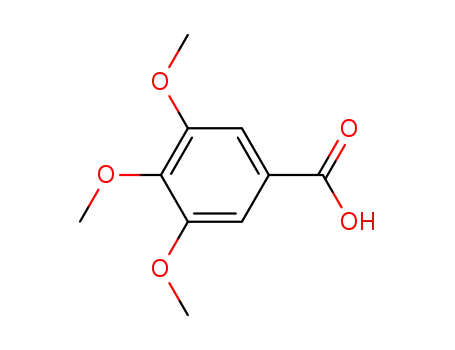
Eudesmic acid

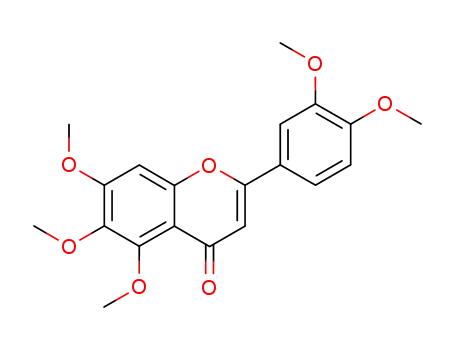
sinensetin
| Conditions | Yield |
|---|---|
|
3,4-dimethoxybenzoic acid chloride; Eudesmic acid;
With
triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
for 2h;
With
trimethylsilyl trifluoromethanesulfonate; triethylamine;
In
dichloromethane;
at 95 ℃;
for 2h;
|
81% |
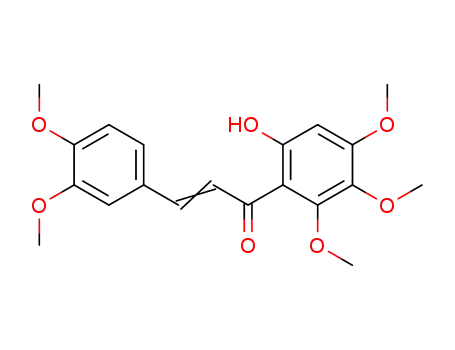
2'-hydroxy-3,4,4',5',6'-pentamethoxychalcone


sinensetin
| Conditions | Yield |
|---|---|
|
With
sulfuric acid;
In
dimethyl sulfoxide;
Heating;
|
76% |
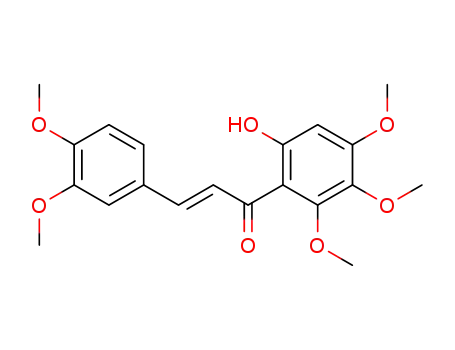
(E)-3-(3,4-dimethoxyphenyl)-1-(6-hydroxy-2,3,4-trimethoxyphenyl)prop-2-en-1-one
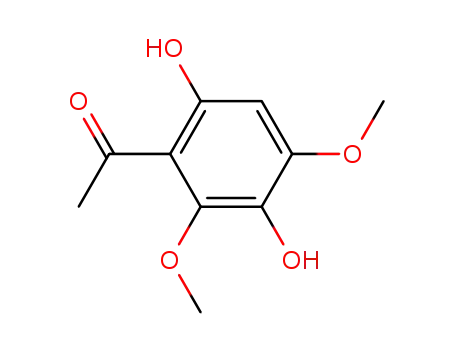
3,6-dihydroxy-2,4-dimethoxyacetophenone
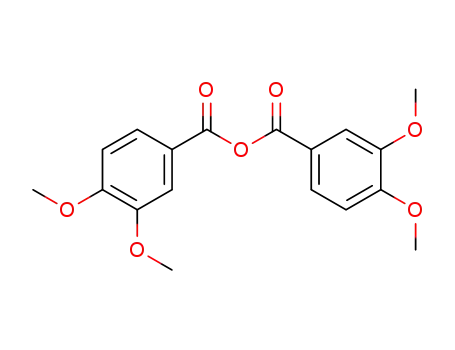
3,4-dimethoxybenzoic anhydride
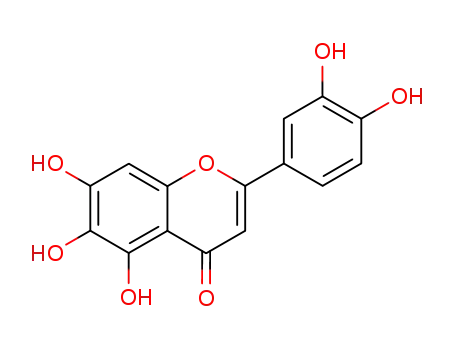
3',4',5,6,7-pentahydroxyflavone

3',4',5,6,7-pentahydroxyflavone
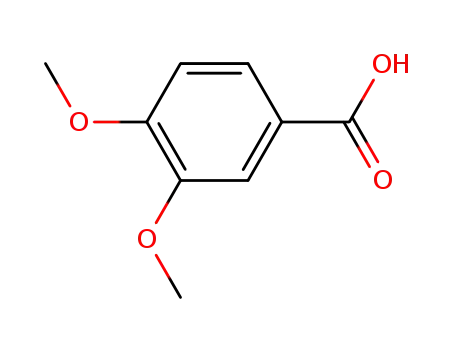
Veratric acid
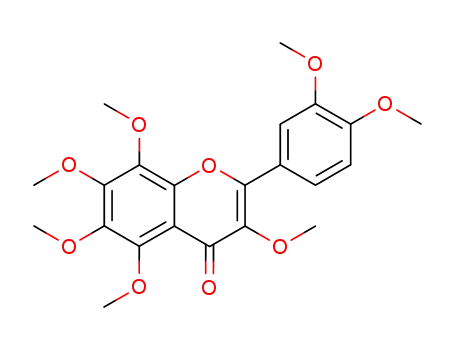
3,3',4',5,6,7,8-heptamethoxyflavone
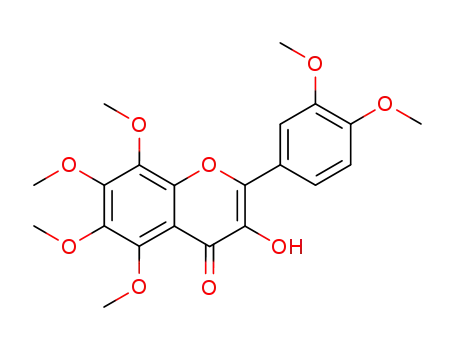
3-hydroxy-5,6,7,8,3′,4′-hexamethoxyflavone
CAS:112163-33-4
CAS:112-34-5
CAS:110-26-9
CAS:1029044-16-3