- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >intermediate >5728-52-9
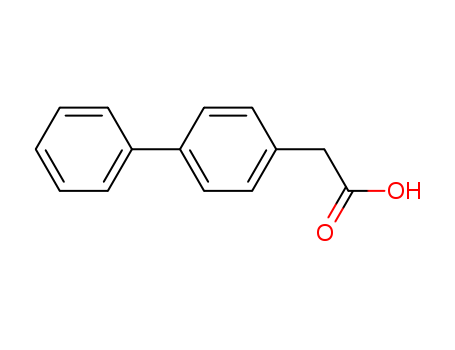

pd_meltingpoint:159-160 °C(lit.)
Appearance:Off-white powder
Purity:99%
|
Safety Profile |
Poison by subcutaneous route.Moderately toxic by ingestion and intraperitoneal routes.An experimental teratogen. Other experimentalreproductive effects. When heated to decomposition itemits acrid smoke and fumes. |
|
Brand name |
Dolinac (Wyeth-Ayerst); Flexfree(Wyeth-Ayerst); Napageln (Wyeth-Ayerst); Target (Wyeth-Ayerst); Traxam (Wyeth-Ayerst). |
|
General Description |
4-Biphenylacetic acid is a potential non-steroidal antiinflammatory agent and forms solid inclusion complex with β-cyclodextrin. 4-Biphenylacetic acid on interaction with quinolone antibacterial agents induces functional blockade of the γ-aminobutyric acid receptors. |
InChI:InChI=1/C14H12O2/c15-14(16)10-11-6-8-13(9-7-11)12-4-2-1-3-5-12/h1-9H,10H2,(H,15,16)/p-1
The simple impregnation of ?3-Fe2O3(core...
We have developed nanoparticle-embedded ...
The development and study of new solvent...
Ceria (CeO2)-supported metal catalysts h...
We have developed a high-efficiency and ...
A highly efficient carboxylation of benz...
It is highly attractive and challenging ...
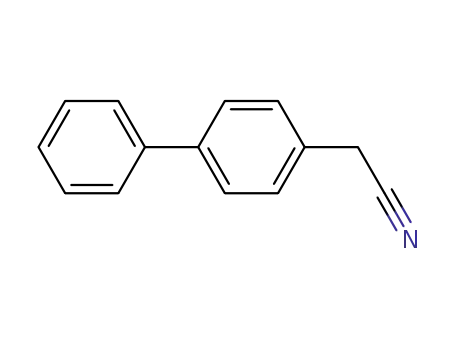
4-biphenylacetonitrile

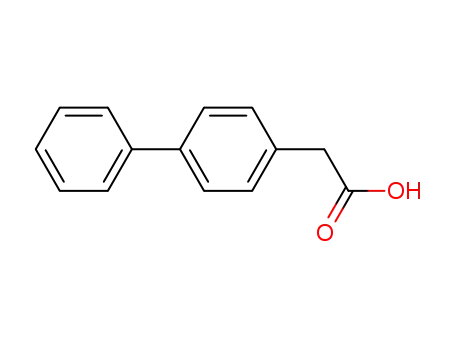
(4-biphenylyl)acetic acid
| Conditions | Yield |
|---|---|
|
With
water; potassium hydroxide;
In
ethylene glycol;
at 120 ℃;
for 16h;
|
97.6% |
|
With
hydrogenchloride;
|
|
|
With
water; sodium hydroxide;
for 4h;
Time;
Reflux;
|
|
|
With
sodium hydroxide;
In
water; butan-1-ol;
at 100 ℃;
for 8h;
Temperature;
|
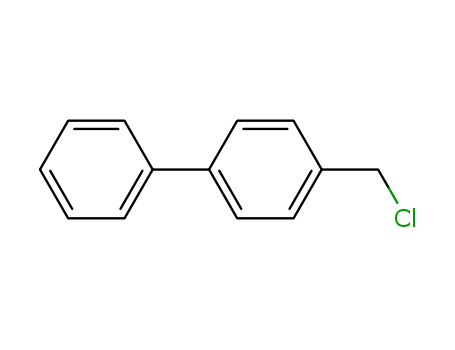
biphenyl-4-methylchloride

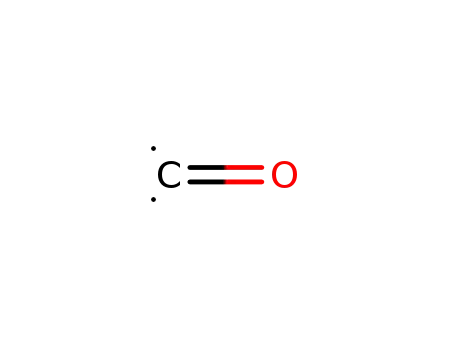
carbon monoxide


(4-biphenylyl)acetic acid
| Conditions | Yield |
|---|---|
|
carbon monoxide;
With
C28H22CoN4O6;
In
butan-1-ol;
at 60 ℃;
for 2h;
under 760.051 Torr;
Glovebox;
High pressure;
Green chemistry;
biphenyl-4-methylchloride;
With
tetra-(n-butyl)ammonium iodide; sodium hydroxide;
In
butan-1-ol;
at 60 ℃;
for 22h;
under 760.051 Torr;
regioselective reaction;
Glovebox;
High pressure;
Green chemistry;
|
80% |
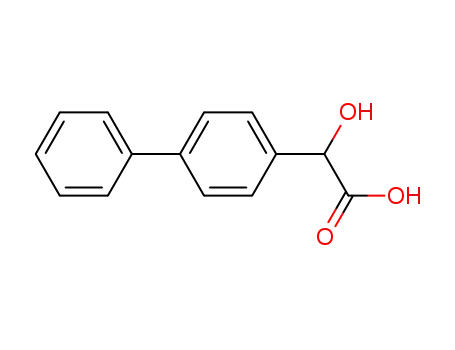
4-phenylmandelic acid
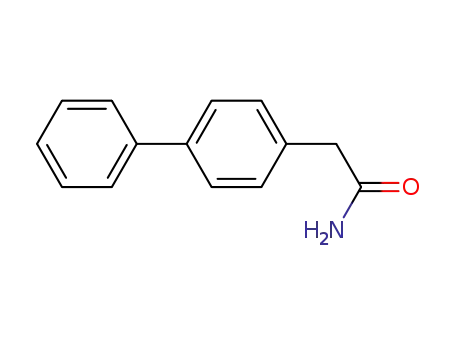
1,1-biphenyl-4-ylacetamide
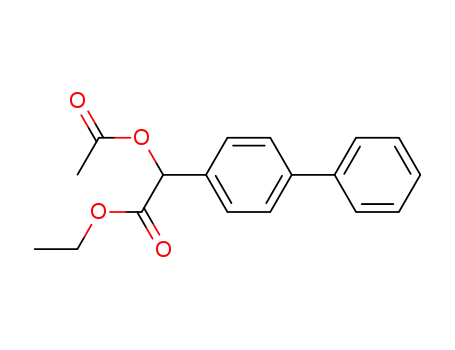
acetoxy-biphenyl-4-yl-acetic acid ethyl ester
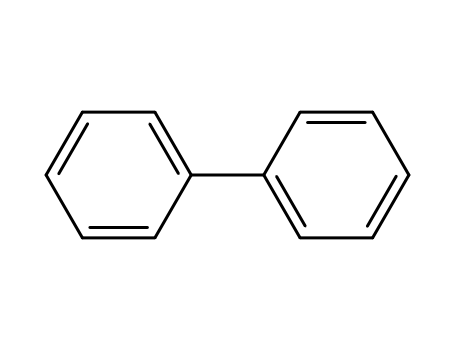
biphenyl
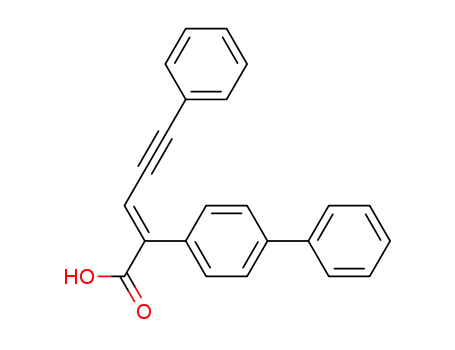
2-biphenyl-4-yl-5-phenyl-pent-2-en-4-ynoic acid
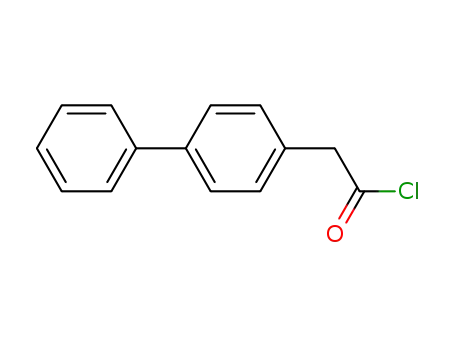
biphenyl-4-yl-acetyl chloride
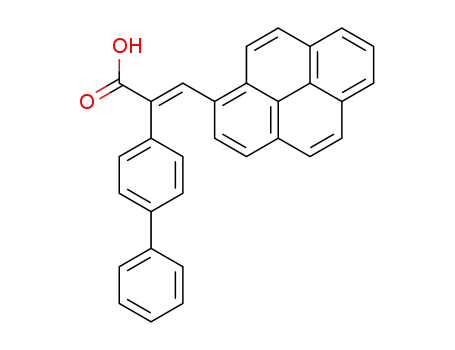
cis-1-
cis-1-
CAS:112163-33-4
CAS:112-34-5
CAS:103060-53-3
CAS:25639-21-8