- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >123653-11-2
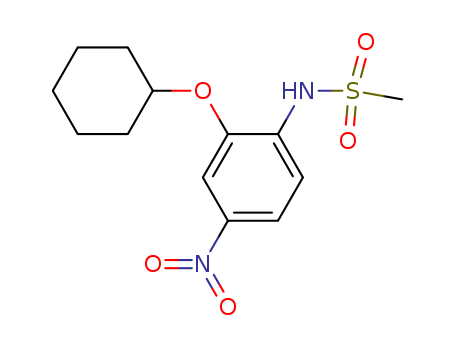

pd_meltingpoint:124-126 °C(Solv: ethyl acetate (141-78-6); hexane (110-54-3))
Purity:99%
|
Biological Activity |
Selective cyclooxygenase-2 inhibitor (IC 50 values are 3.8 and > 100 μ M for COX-2 and COX-1 respectively). Induces apoptosis in colorectal tumor cells and elevates COX-2 protein expression in vitro . Orally active and non-ulcerogenic analgesic and anti-inflammatory in vivo . |
|
references |
[1]. futaki n, takahashi s, yokoyama m, et al. ns-398, a new anti-inflammatory agent, selectively inhibits prostaglandin g/h synthase/cyclooxygenase (cox-2) activity in vitro. prostaglandins, 1994, 47(1): 55-59.[2]. elder dj, halton de, crew te, et al. apoptosis induction and cyclooxygenase-2 regulation in human colorectal adenoma and carcinoma cell lines by the cyclooxygenase-2-selective non-steroidal anti-inflammatory drug ns-398. int j cancer, 2000, 86(4): 553-560.[3]. mack strong ve, mackrell pj, concannon em, et al. ns-398 treatment after trauma modifies nf-kappab activation and improves survival. j surg res, 2001, 98(1): 40-46. |
|
Definition |
ChEBI: A C-nitro compound that is N-methylsulfonyl-4-nitroaniline bearing an additional cyclohexyloxy substituent at position 2. |
InChI:InChI=1/C13H18N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h7-9,11,14H,2-6H2,1H3
Compounds and methods suppressing aromat...
Aromatase is a particularly good target ...
Aromatase is a particularly attractive t...
Cachexia, including anorexia and other f...
C14H20N2O7S2

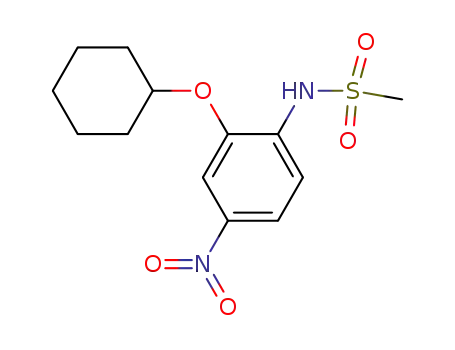
N-(2-cyclohexyloxy-4-nitrophenyl)methanesulfonamide
| Conditions | Yield |
|---|---|
|
C14H20N2O7S2;
With
sodium hydroxide; water;
at 80 - 90 ℃;
With
hydrogenchloride;
In
water;
pH=1 - 2;
|
59.5% |
|
With
potassium hydroxide;
for 2h;
Heating;
|
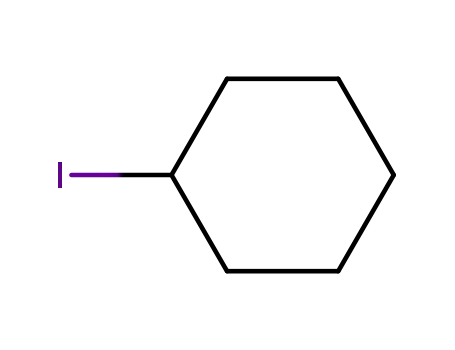
Cyclohexyl iodide


N-(2-cyclohexyloxy-4-nitrophenyl)methanesulfonamide
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1.1: 5 percent / K2CO3 / dimethylformamide / 120 h / Heating
2.1: NaH / dimethylformamide / 0.33 h
2.2: 20 °C
3.1: aq. KOH / 2 h / Heating
With
potassium hydroxide; sodium hydride; potassium carbonate;
In
N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1.1: 6.5 percent / K2CO3 / dimethylformamide / 168 h / Heating
2.1: NaH / dimethylformamide / 0.5 h / 20 °C
2.2: dimethylformamide / 20 °C
2.3: 59.5 percent / aq. NaOH / 80 - 90 °C
With
sodium hydride; potassium carbonate;
In
N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1.1: potassium carbonate / N,N-dimethyl-formamide / 168 h / Heating / reflux
2.1: sodium hydride / N,N-dimethyl-formamide / 0.5 h / 20 °C
2.2: 20 °C
2.3: pH 1 - 2
3.1: sodium hydroxide; water / 80 - 90 °C
3.2: pH 1 - 2
With
sodium hydroxide; water; sodium hydride; potassium carbonate;
In
N,N-dimethyl-formamide;
|
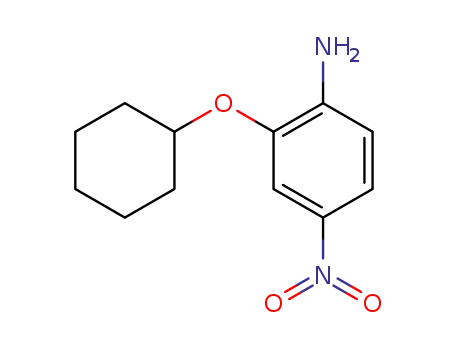
2-cyclohexyloxy-4-nitroaniline
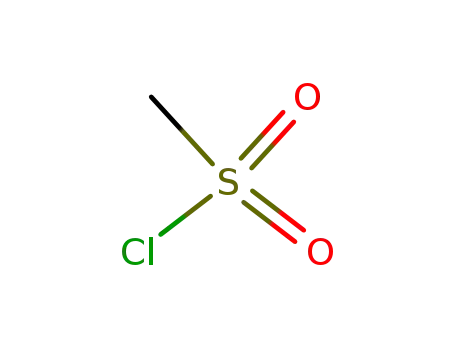
methanesulfonyl chloride
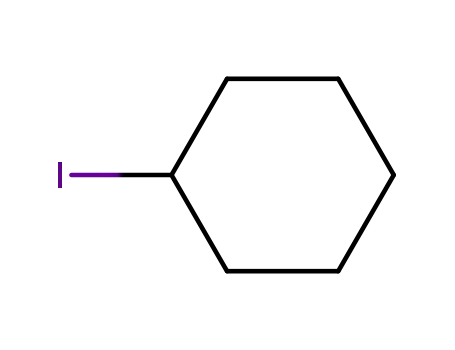
Cyclohexyl iodide
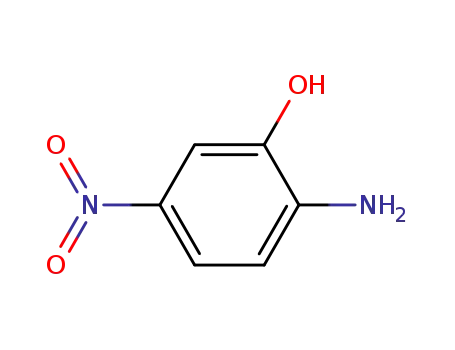
5-Nitro-2-aminophenol
CAS:115473-15-9
CAS:1173-88-2
CAS:733750-99-7
CAS:113852-37-2