- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >105650-23-5
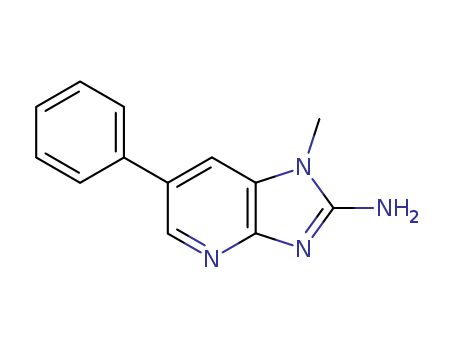

pd_meltingpoint:300 °C
Appearance:Off-white solid
Purity:99%
|
Safety Profile |
Confirmed carcinogen with experimental carcinogenic data. Mutation data reported. When heated to decomposition it emits toxic vapors of NOx,. |
|
Carcinogenicity |
PhIP is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals and supporting genotoxicity data. |
|
Definition |
ChEBI: An imidazopyridine that is 1H--imidazo[4,5-b]pyridine which is substituted at positions 1, 2, and 6 by methyl, amino, and phenyl groups, respectively. It is the most abundant of the mutagenic heterocyclic amines found in coo ed meat and fish. |
InChI:InChI=1/C13H12N4/c1-17-11-7-10(9-5-3-2-4-6-9)8-15-12(11)16-13(17)14/h2-8H,1H3,(H2,14,15,16)
Base-induced cyclization of N′-cyanometh...
In situ generation of efficient carbonyl...
There is a correlation between intestina...
A modified synthetic approach to the syn...
(Chemical Equation Presented) Synthesis ...
![1-methyl-6-phenyl-1H-imidazo[4,5-b]pyridine](/upload/2025/4/ce1e6caa-a3a1-47f8-bab1-6e5b77dfcac7.png)
1-methyl-6-phenyl-1H-imidazo[4,5-b]pyridine

![2-amino-1-methyl-6-phenyl-1H-imidazo[4,5-b]pyridine](/upload/2025/4/cdb228cc-5499-4faa-bdc5-65ed6a5fa5c6.png)
2-amino-1-methyl-6-phenyl-1H-imidazo[4,5-b]pyridine
| Conditions | Yield |
|---|---|
|
With sodium amide; In xylene; at 130 ℃; for 1.5h;
|
90.2% |
|
With sodium amide;
|
80% |
|
With 15-crown-5; sodium amide; In xylene; at 130 ℃; for 1.5h;
|
20 % Turnov. |
![2-amino-1-methylbenzothieno[3,2-e]imidazo[4,5-b]pyridine](/upload/2025/4/bfc43452-4897-4a28-9ab8-123ae8fc1e75.png)
2-amino-1-methylbenzothieno[3,2-e]imidazo[4,5-b]pyridine

![2-amino-1-methyl-6-phenyl-1H-imidazo[4,5-b]pyridine](/upload/2025/4/cdb228cc-5499-4faa-bdc5-65ed6a5fa5c6.png)
2-amino-1-methyl-6-phenyl-1H-imidazo[4,5-b]pyridine
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; nickel; In 1,4-dioxane; ethanol; Heating;
|
35% |
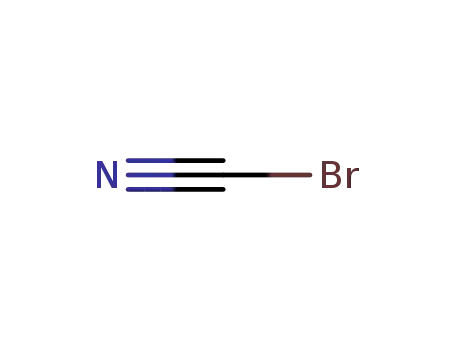
bromocyane
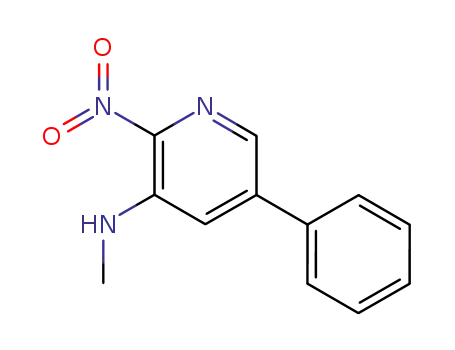
3-methylamino-2-nitro-5-phenylpyridine
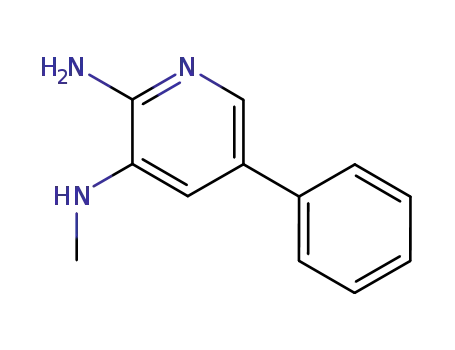
2-amino-3-methylamino-5-phenylpyridine
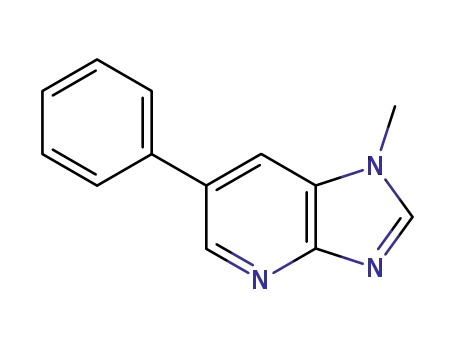
1-methyl-6-phenyl-1H-imidazo[4,5-b]pyridine
CAS:112163-33-4
CAS:112-84-5
CAS:366017-09-6
CAS:1316311-27-9