- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
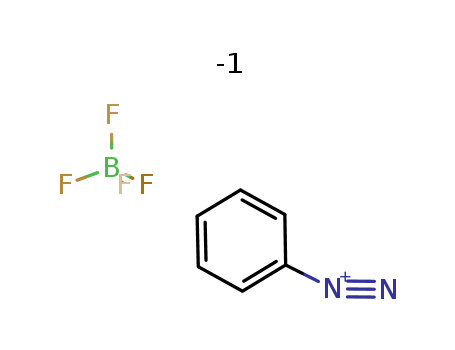
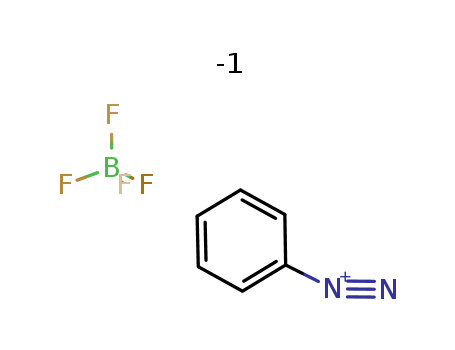
pd_meltingpoint:121-122 °C (decomp)
Purity:99%
|
Safety Profile |
Poison by ingestion andsubcutaneous routes. Questionable carcinogen withexperimental tumorigenic data. Mutation data reported.When heated to decomposition it emits toxic fumes ofNOx and F-. |
InChI:InChI=1/C6H5N2.BF4/c7-8-6-4-2-1-3-5-6;2-1(3,4)5/h1-5H;/q+1;-1
Five organic redox systems were examined...
The title compound, C18H19N3O2, was obta...
Porous organic polymers (POPs) have rece...
Direct C5 (hetero)arylation of uracil an...
We report a modular approach toward nove...
The first metal-free procedure for the s...
Given their ubiquity in natural products...
tetrafluoroboric acid

aniline

benzenediazonium tetrafluoroborate
| Conditions | Yield |
|---|---|
|
With sodium nitrite; In water; at 0 ℃; for 3h;
|
99.5% |
|
tetrafluoroboric acid; aniline; In ethanol; water; at 20 ℃; for 0.0333333h;
With tert.-butylnitrite; In ethanol; water; at 0 - 20 ℃; for 1.25h;
|
98% |
|
tetrafluoroboric acid; aniline; In ethanol; water; for 0.25h;
With sodium nitrite; In ethanol; water; at 0 ℃; for 1h;
|
98% |
|
With sodium nitrite; In water; at 0 ℃; for 1h; Inert atmosphere;
|
93% |
|
With sodium nitrite; In water; at -7 - 1 ℃; for 2h;
|
87% |
|
With sodium nitrite; In water; at 0 - 5 ℃; for 2h;
|
86.6% |
|
With sodium nitrite; In water; for 0.166667h;
|
86.7% |
|
aniline; With hydrogenchloride; sodium nitrite; In water; at 0 ℃; for 0.5h;
tetrafluoroboric acid; In water; at 0 ℃; Cooling with ice;
|
85% |
|
With tert-butyl methyl ether; sodium nitrite; In water; at 0 - 20 ℃; for 0.833333h; Inert atmosphere;
|
80% |
|
tetrafluoroboric acid; aniline; In water; at 0 ℃; for 1h;
With sodium nitrite; In water; for 1h;
|
76% |
|
With sodium nitrite; In water; at 0 ℃; for 1h;
|
74% |
|
With sodium nitrite; In water; at 0 ℃; for 0.5h;
|
70% |
|
tetrafluoroboric acid; aniline; In water; at 0 ℃;
With sodium nitrite; In water; at 0 ℃; for 0.5h;
|
60% |
|
With sodium nitrite; In water; at 0 ℃; for 0.666667h;
|
49% |
|
With sodium nitrite; In water; at -5 - 0 ℃; for 0.666667h;
|
|
|
With sodium nitrite; In water; for 0.5h; Cooling with ice;
|
|
|
With sodium nitrite; In water; for 0.5h; Cooling with ice;
|
|
|
With sodium nitrite; In water; at 0 ℃;
|
|
|
With sodium nitrite; In water; at 0 ℃; for 3.33333h;
|
|
|
With tert.-butylnitrite; In ethanol; water; at 0 - 20 ℃; for 1h;
|
|
|
With sodium nitrite; In water; at 0 ℃; for 0.666667h; Inert atmosphere; Schlenk technique;
|
|
|
With sodium nitrite; In water; at 0 ℃; for 0.666667h;
|
|
|
With sodium nitrite; In water; for 0.5h; Cooling with ice;
|
|
|
With tert.-butylnitrite; In ethanol; water; at 0 - 20 ℃; for 1h;
|
|
|
With sodium nitrite; In water; at 0 ℃; for 1h;
|
|
|
tetrafluoroboric acid; aniline; In ethanol; water; at 20 ℃; for 0.0833333h;
With tert.-butylnitrite; In ethanol; water; at 0 ℃; for 2h; Inert atmosphere;
|
|
|
With sodium nitrite; In water; at 0 ℃; for 0.5h;
|
boron trifluoride diethyl etherate


aniline

benzenediazonium tetrafluoroborate
| Conditions | Yield |
|---|---|
|
With tert.-butylnitrite; In dichloromethane; at 0 - 20 ℃; for 1h; Inert atmosphere;
|
95% |
|
With tert.-butylnitrite; In dichloromethane; at 0 - 20 ℃; for 2h;
|
|
|
With tert.-butylnitrite; In methanol; at -20 - 20 ℃; Inert atmosphere;
|
phenylhydrazine hydrochloride

aniline
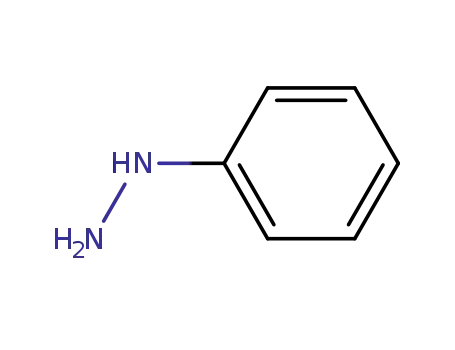
phenylhydrazine
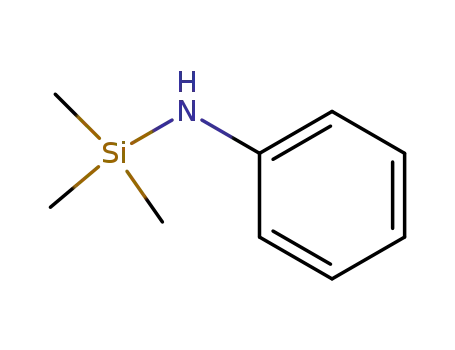
N-trimethylsilylaniline
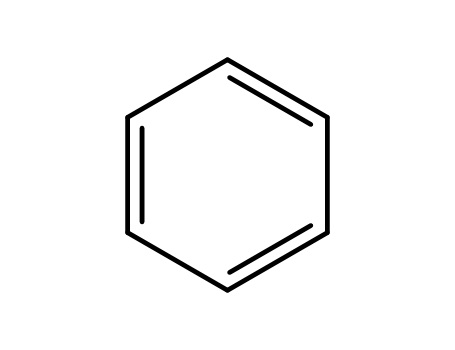
benzene
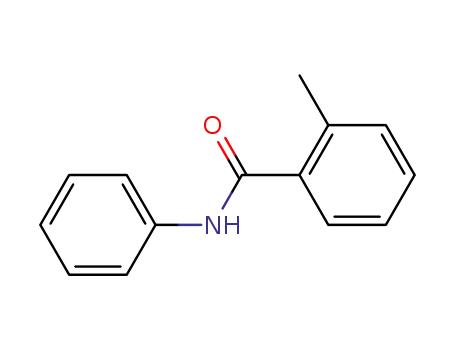
2-methyl-N-phenylbenzamide
fluorobenzene

phenyl radical
CAS:850140-73-7
CAS:112163-33-4
CAS:136572-09-3
CAS:1629229-37-3