- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >850140-73-7

Purity:99%
The invention discloses a preparation me...
The invention provides a novel preparati...
The invention belongs to the technical f...
The invention relates to a novel afatini...
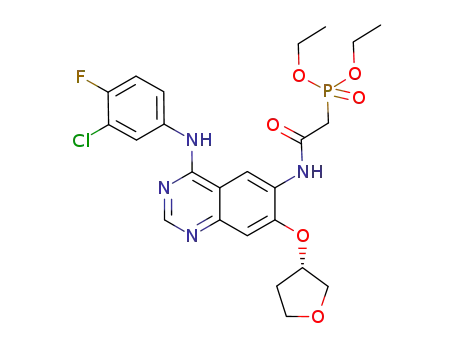
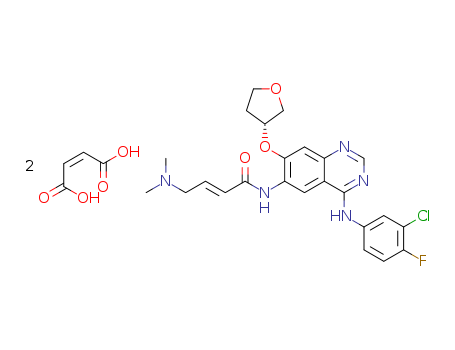
(S)-diethyl 2-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahydrofuran-3-yloxy)quinazolin-6-ylamino)-2-oxoethylphosphonate

![2-butenamide, N-(4-((3-chloro-4-fluorophenyl)amino)-7-([(3S)-tetrahydro-3-furanyl]oxy)-6-quinazolinyl)-4-(dimethylamino)-, (2E)-, (2Z)-2-butenedioate](/upload/2025/4/919a6359-8dde-462e-92fe-61cc6050ed7e.png)
2-butenamide, N-(4-((3-chloro-4-fluorophenyl)amino)-7-([(3S)-tetrahydro-3-furanyl]oxy)-6-quinazolinyl)-4-(dimethylamino)-, (2E)-, (2Z)-2-butenedioate
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1.1: hydrogenchloride / water / 20 - 30 °C / Inert atmosphere
1.2: 2.5 h / -15 - 20 °C / Inert atmosphere
2.1: tetrahydrofuran / 1.03 h / 20 °C
With hydrogenchloride; In tetrahydrofuran; water;
|

(S)-diethyl 2-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahydrofuran-3-yloxy)quinazolin-6-ylamino)-2-oxoethylphosphonate

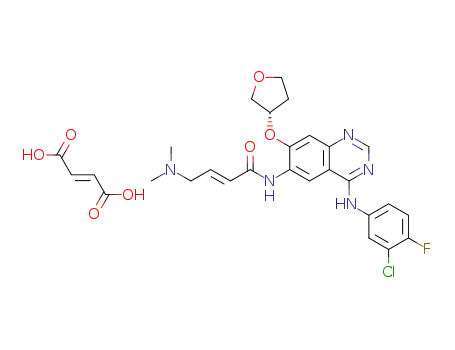
afatinib fumarate
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1.1: hydrogenchloride / water / 20 - 30 °C / Inert atmosphere
1.2: 2.5 h / -15 - 20 °C / Inert atmosphere
2.1: ethanol / 20 - 70 °C
With hydrogenchloride; In ethanol; water;
|
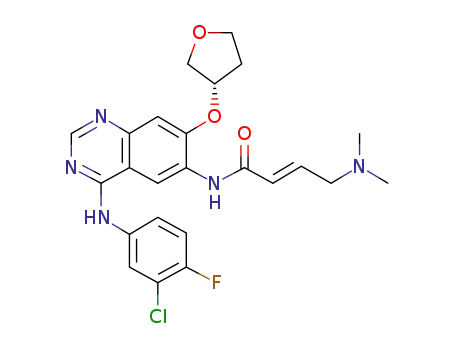
afatinib
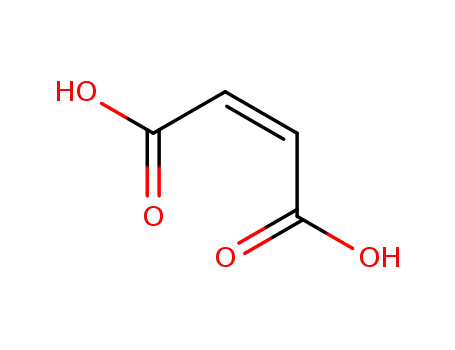
maleic acid

(S)-diethyl 2-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahydrofuran-3-yloxy)quinazolin-6-ylamino)-2-oxoethylphosphonate
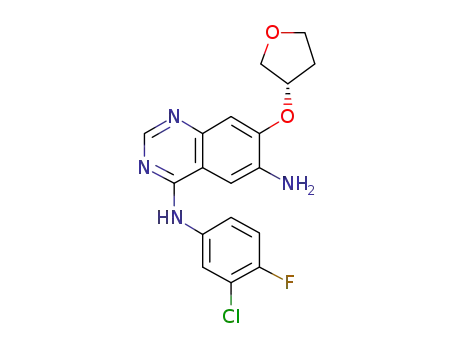
6-Amino-4-[(3-chloro-4-fluorophenyl)amino]-7-[(S)-(tetrahydrofuran-3-yl)oxy]quinazoline
CAS:755037-03-7
CAS:35123-06-9