- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >116496-76-5
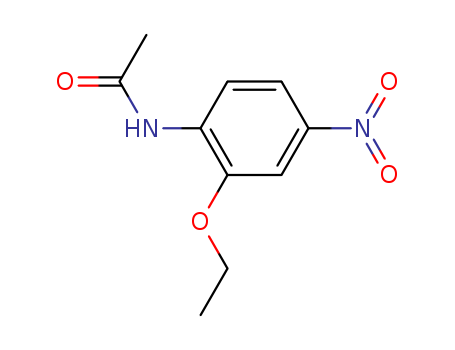

pd_meltingpoint:164-165 °C
Purity:99%
|
General Description |
4-ACETAMIDO-3-ETHOXYNITROBENZENE is a chemical compound that contains a nitro group, an acetamide group, and an ethoxy group attached to a benzene ring. It is often used in organic synthesis and research as a precursor for the production of other compounds. The nitro group makes it a potential precursor for the synthesis of various organic compounds, while the acetamide and ethoxy groups provide additional functionality for potential applications in pharmaceuticals, agrochemicals, and materials science. 4-ACETAMIDO-3-ETHOXYNITROBENZENE is a versatile chemical that has potential uses in a wide range of fields. |
InChI:InChI=1/C10H12N2O4/c1-3-16-10-6-8(12(14)15)4-5-9(10)11-7(2)13/h4-6H,3H2,1-2H3,(H,11,13)
The discovery and development of a novel...
Irreversible epidermal growth factor rec...
New route for the preparation of N-(3-cy...
A series of new 6,7-disubstituted-4-(ary...
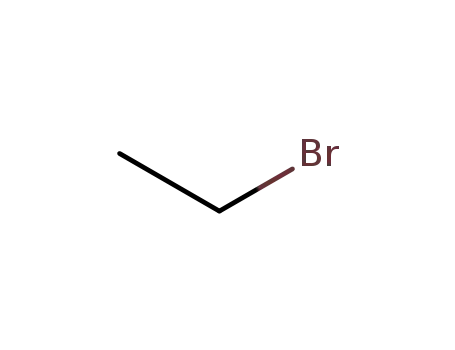
ethyl bromide

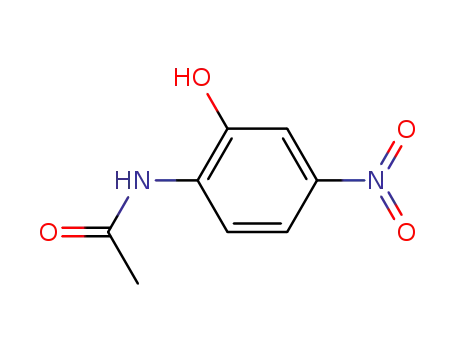
N-(2-hydroxy-4-nitrophenyl)acetamide

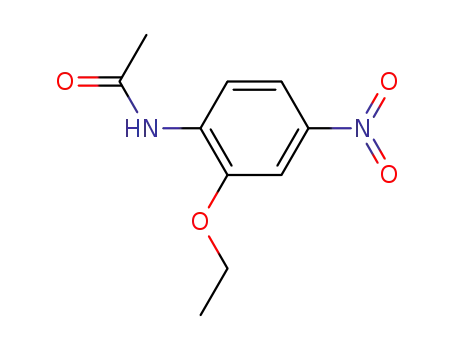
4-acetamido-3-ethoxynitrobenzene
| Conditions | Yield |
|---|---|
|
With potassium carbonate; In N,N-dimethyl-formamide; at 60 ℃;
|
98% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 60 ℃; for 1h;
|
98% |
|
With potassium carbonate; In DMF (N,N-dimethyl-formamide); at 60 ℃; for 2.5h;
|
|
|
With potassium carbonate; In DMF (N,N-dimethyl-formamide); at 20 - 60 ℃; for 2.5h; Heating / reflux;
|
|
|
With potassium carbonate; In N,N-dimethyl-formamide; at 60 ℃;
|
|
|
With potassium carbonate; In N,N-dimethyl-formamide; at 60 ℃;
|
|
|
With potassium carbonate; In N,N-dimethyl-formamide; at 60 ℃;
|
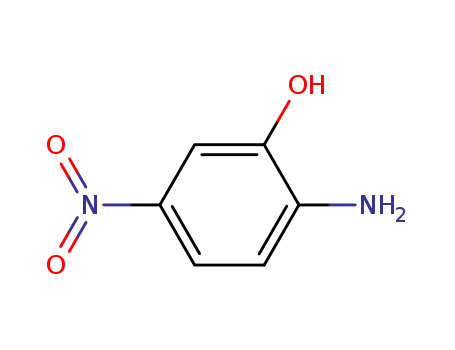
5-Nitro-2-aminophenol


4-acetamido-3-ethoxynitrobenzene
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 96 percent / AcOH / 60 °C
2: 98 percent / K2CO3 / dimethylformamide / 60 °C
With potassium carbonate; acetic acid; In N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1: acetic acid / 60 °C
2: potassium carbonate / N,N-dimethyl-formamide / 60 °C
With potassium carbonate; acetic acid; In N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1: acetic acid / 60 °C
2: potassium carbonate / N,N-dimethyl-formamide / 60 °C
With potassium carbonate; acetic acid; In N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1: acetic acid / 1 h / 60 °C
2: potassium carbonate / N,N-dimethyl-formamide / 60 °C
With potassium carbonate; In acetic acid; N,N-dimethyl-formamide;
|
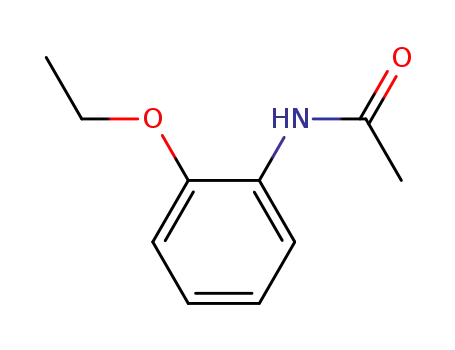
2'-ethoxyacetanilide
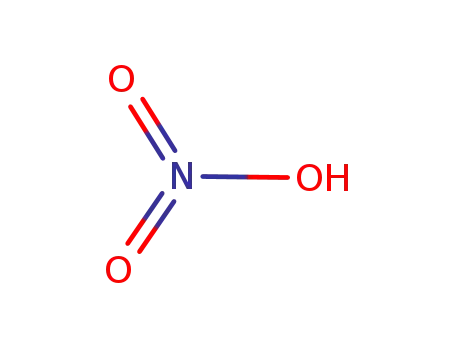
nitric acid
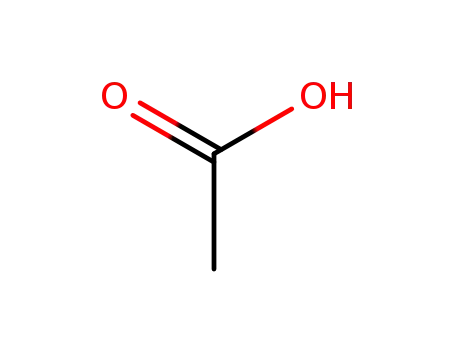
acetic acid
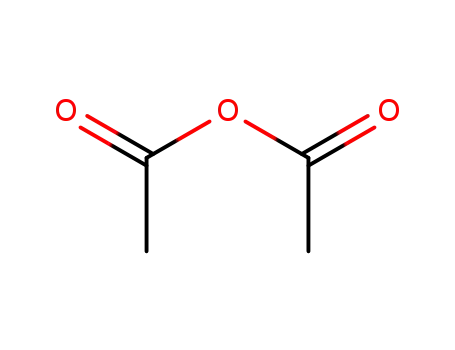
acetic anhydride
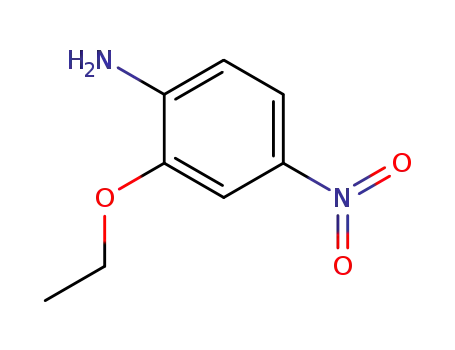
5-nitro-2-amino-phenetole
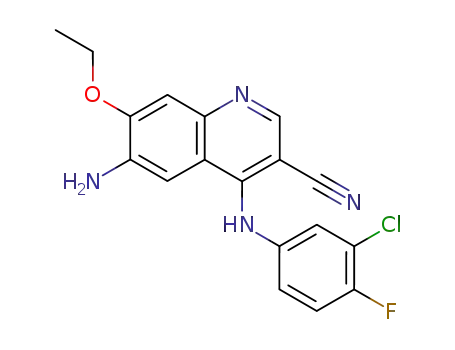
6-amino-4-(3-chloro-4-fluoro-phenylamino)-7-ethoxy-quinoline-3-carbonitrile
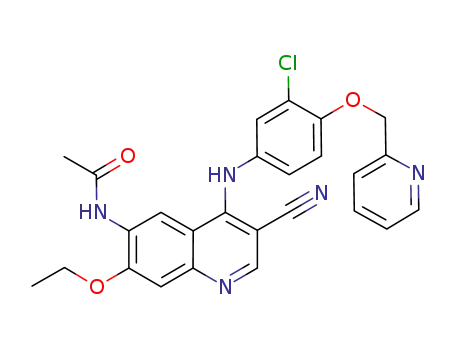
N-(4-[3-chloro-4-(pyridin-2-ylmethoxy)phenylamino]-3-cyano-7-ethoxyquinoline-6-yl)acetamide
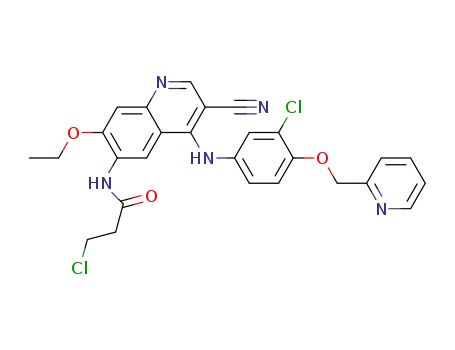
3-chloro-N-(4-(3-chloro-4-(pyridin-2-ylmethoxy)anilino)-3-cyano-7-ethoxyquinolin-6-yl)propanamide
CAS:112163-33-4
CAS:112-84-5
CAS:1258419-69-0
CAS:321674-73-1