- +86-0533-2185556
- +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >321674-73-1
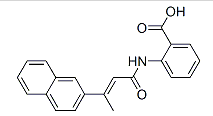

Purity:99%
|
Biological Activity |
Selective telomerase inhibitor (IC 50 values are 93, > 100000 and > 100000 nM for human telomerase, human RNA polymerase I and human RNA polymerase II + III respectively). Causes telomere shortening in exponentially growing NCI-H460 lung carcinoma cells and eventual growth arrest. |
|
references |
[1]. damm, k.; hemmann, u.; garin-chesa, p.; hauel, n.; kauffman, i.; priepke, h.; niestroj, c.; daiber, c.; enenkel, b.; guilliard, b.; lauritsch, i.; muller, e.; pascolo, e.; sauter, g.; pantic, m.; martens, u. m.; wenz, c.; linger, j.; kraut, n.; rettig, w. j.;schnapp, a. a highly selective telomerase inhibitor limiting human cancer cell proliferation. embo j. 2001, 20, 6958?6968.[2]. bashash d1, ghaffari sh, mirzaee r, alimoghaddam k, ghavamzadeh a. telomerase inhibition by non-nucleosidic compound bibr1532 causes rapid cell death in pre-b acute lymphoblastic leukemia cells. leuk lymphoma. 2013 mar;54[4]:561-8. doi: 10.3109/10428194.2012.704034. epub 2012 sep 28.[3]. bashash d1, ghaffari sh, zaker f, kazerani m, hezave k, hassani s, rostami m, alimoghaddam k, ghavamzadeh a. anticancer agents med chem. 2013 sep;13(7):1115-25. bibr 1532 increases arsenic trioxide-mediated apoptosis in acute promyelocytic leukemia cells: therapeutic potential for apl. |
InChI:InChI=1/C21H17NO3/c1-14(16-11-10-15-6-2-3-7-17(15)13-16)12-20(23)22-19-9-5-4-8-18(19)21(24)25/h2-13H,1H3,(H,22,23)(H,24,25)/b14-12+
The present disclosure provides TERT inh...
The invention relates to N-substituted a...
BIBR 1532 has been reported to be a pote...
Carboxylic acid amides of general formul...
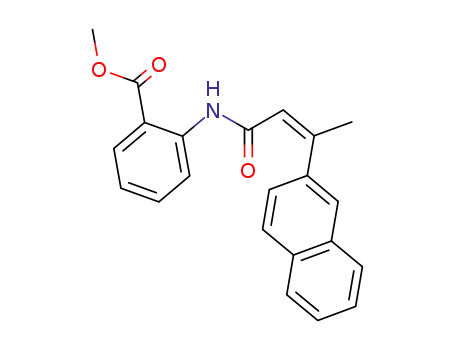
N-(2-carbomethoxyphenyl)-3-(naphthalen-2-yl)-but-2(Z)-enamide

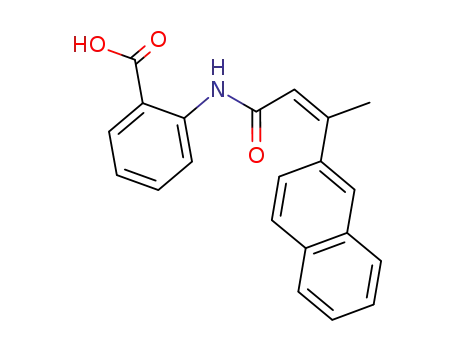
trans-3-(naphth-2-yl)-but-2-enoic acid-N-(2-carboxy-phenyl)-amide
| Conditions | Yield |
|---|---|
|
With
lithium hydroxide;
In
tetrahydrofuran;
at 20 ℃;
for 12h;
|
|
|
With
sodium hydroxide;
In
ethanol;
|
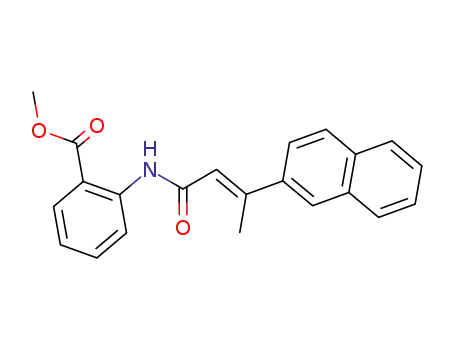
N-(2-carbomethoxyphenyl)-3-(naphthalen-2-yl)-but-2(E)-enamide

![2-[[(2E)-3-(2-naphthalenyl)-1-oxo-2-butenyl-1-yl]amino]benzoic acid](/upload/2025/4/d0243743-7533-4ea1-9aa0-6a6d1e40baf3.png)
2-[[(2E)-3-(2-naphthalenyl)-1-oxo-2-butenyl-1-yl]amino]benzoic acid
| Conditions | Yield |
|---|---|
|
With
lithium hydroxide;
In
tetrahydrofuran;
at 20 ℃;
for 12h;
|
|
|
With
water; lithium hydroxide;
In
tetrahydrofuran; methanol;
|

N-(2-carbomethoxyphenyl)-3-(naphthalen-2-yl)-but-2(Z)-enamide
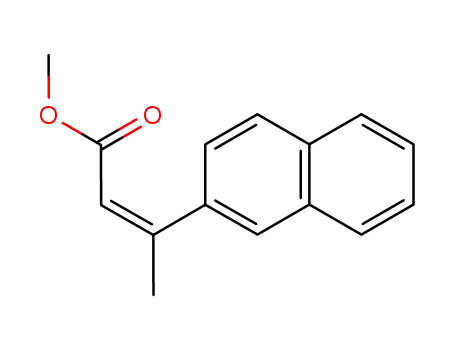
methyl (Z)-3-(naphthalen-2-yl)-but-2-enoate
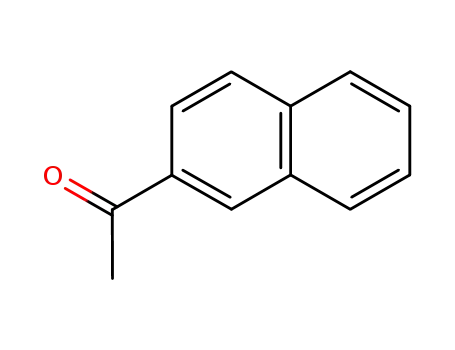
methyl 2-naphthyl ketone

N-(2-carbomethoxyphenyl)-3-(naphthalen-2-yl)-but-2(E)-enamide
CAS:112163-33-4
CAS:112-84-5
CAS:116496-76-5
CAS:1374516-07-0